 This week we spoke with Dr Matt Salamonsen about the specialty – Interventional Pulmonology.
This week we spoke with Dr Matt Salamonsen about the specialty – Interventional Pulmonology.
Interventional pulmonology: What is it and where does it fit in modern clinical practice?
Interventional pulmonology is a relatively young but rapidly growing sub-specialty of respiratory medicine. While traditional respiratory physicians have performed basic flexible bronchoscopy, interventional pulmonology encompasses a range of more advanced bronchoscopic and pleural procedures, which have emerged with new technologies over the last 10-15 years.
Diagnostic bronchoscopy
- Flexible bronchoscopy
- Endobronchial Ultrasound (EBUS) guided biopsy of mediastinal or lung pathology
- Transbronchial cryobiopsy
Interventional bronchoscopy
- Rigid bronchoscopy
- Management of central airway obstruction (malignant or benign)
- Endobronchial laser and stenting
- Endobronchial valves for lung volume reduction or prolonged air-leak
- Bronchial thermoplasty for asthma
Pleural
- Thoracentesis, chest tubes, pleurodesis
- Tunnelled pleural catheter placement
- Pleuroscopy (Medical thoracoscopy)
Table 1 Interventional pulmonology procedures
Endobronchial Ultrasound: EBUS
EBUS, a method to obtain biopsies from mediastinal structures adjacent to the central airways or in the periphery of the lung, has been a ‘game-changing’ technology and is now the standard of care world-wide.
Initially developed to diagnose end stage lung cancer (see below), it now has a proven role in the diagnosis of any pathology affecting the mediastinum, mediastinal lymph nodes, hilar structures or lung parenchyma. Common examples would include: lung cancer, metastatic cancer, lymphoma, sarcoidosis, tuberculosis and peripheral pulmonary nodules.
There are two types of EBUS: Linear EBUS (to sample mediastinal or hilar structures adjacent to the central airways) and radial EBUS (to locate and sample parenchymal lung lesions). These use different technologies and are completely different procedures – see Figure 1.
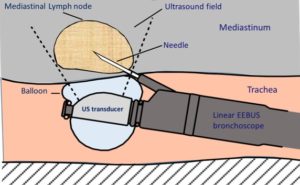
Lung cancer
A small word on lung cancer as it accounts for the lion’s share of work in interventional pulmonology.
There are 2 critical steps in evaluating lung cancer. The first is to obtain a biopsy to define the tissue type (adenocarcinoma vs small cell carcinoma etc) and the second is to accurately stage the cancer by identifying which mediastinal lymph nodes or extra-thoracic sites contain cancer. Staging mediastinal lymph node spread is critical as depending on which nodes are affected, the stage can range from early amenable to surgical resection to advanced with palliative chemoradiotherapy the only option (See figure 2).
PET scan is useful to screen for extra-thoracic disease, but its sensitivity and specificity is inadequate to accurately stage the mediastinal lymph nodes in many cases. Soley relying on PET (which has widely been the practice) will lead to a significant number of people with advanced disease inappropriately undergoing surgical resection, and the denial of curative surgery to a significant number with more limited disease. EBUS staging of the mediastinal lymph nodes offers a minimally invasive technique to accurately assess disease extent. It has changed the paradigm for the diagnosis and staging of lung cancer and is now the standard of care worldwide.
Transbronchial cryobiopsy
Transbronchial cryobiopsy is an emerging technique which allows larger biopsies (diameter 6-10mm) to be taken from the lung parenchyma than obtained by traditional forceps biopsy (diameter ~1mm). A cryoprobe inserted through the working channel of a flexible bronchoscope is passed into the lung periphery. Once positioned, the cryoprobe is activated, reducing the temperature at the tip to -59oC which freezes a ball of lung parenchyma onto the probe, before it is withdrawn extracting the biopsy – see Figure 2.
Transbronchial cryobiopsy has a much higher yield than traditional forceps biopsy for the diagnosis of lung parenchymal diseases (eg pulmonary fibrosis, sarcoidosis). How it compares to surgical lung biopsy is unknown (a multi-centre trial of cryobiopsy vs surgical biopsy is currently underway). Most likely it will find its place as the first, less invasive method for obtaining a lung biopsy in parenchymal lung diseases, leaving surgical biopsy to those in which cryobiopsy is inadequate.
Importantly, as much larger biopsies are being taken from the lung, the risk of major haemorrhage is greatly increased over forceps biopsy. Safe performance of transbronchial cryobiopsy requires the prophylactic placement of an endbronchial balloon that can be used to tamponade major haemorrhage if it occurs. This necessitates the procedure to be performed according to a carefully controlled protocol, ideally via rigid bronchoscopy, or at a minimum an endotracheal tube.
Rigid bronchoscopy, laser and stenting
Rigid bronchoscopy is an indispensable tool for the interventional pulmonologist. It provides unrestricted access to the central airways and allows multiple tools to be employed simultaneously. These include: large suction catheters (to deal with significant haemorrhage), rigid forceps (for removal of foreign bodies or tumour tissue), laser or electrocautery to coagulate tissue for hemostasis or devitalisation of tumour tissue, and stent deployment catheters. The barrel of the rigid bronchoscope can be used for dilating stenoses or coring-out endobronchial tumour. A flexible bronchoscope can also be passed through the rigid bronchoscope to perform more routine procedures.
Procedures performed via rigid bronchoscopy include:
- Management of malignant central airway obstruction (coring of tumour, dilatation stenoses +/- insertion of stent)
- Mangement of benign central airways obstruction (dilatation +/- stent)
- Other diseases requiring stent insertion (eg tracheobronchomalacia, bronchopleural fistula)
- Application of laser or electrocautery to control haemoptysis from central airway pathology.
- Removal of foreign bodies
- Transbronchial cryobiopsy
Endobronchial valves for lung volume reduction or management of persistent air leaks
Severe emphysema is a debilitating condition that results in a very poor quality of life. In patients where significant hyperinflation of emphysematous lung compresses relatively healthy lung, lung volume reduction with removal of the most emphysematous regions can result in significant symptomatic benefit. Surgical lung volume reduction in these patients has been shown to significantly improve exercise tolerance and quality of life, but is associated with a high rate of adverse events and mortality. Bronchoscopic lung volume reduction has been developed in an effort to obtain the same symptomatic benefit, while avoiding the high mortality/morbidity.
Bronchoscopic lung volume reduction is performed by inserting one-way valves to occlude segmental airways of the target lung lobe (hyper-inflated emphysematous region). This results in atelectasis of this hyper-inflated lobe with expansion of the relatively healthy lung – see Figure 3. There is now good evidence to support this procedure in the subset of emphysema patients with severe hyperinflation, and it has been included in the most recent GOLD (Global initiative on Obstructive Lung Disease) guidelines.
Endobronchial valves can also be used to seal a persistent airleak (bronchopleural/parenchymopleural fistula) such as may occur with secondary spontaneous pneumothorax or post thoracic surgery. The culprit lung lobe is identified by systematically inflating a balloon endobronchially to block lobar bronchi and observing when bubbling from the chest tube drain ceases. Endobronchial valves are then inserted to occlude these lobar bronchi, sealing the air leak.
Bronchial thermoplasty for severe asthma
Bronchial thermoplasty is a procedure where thermal energy is applied to the walls of proximal lobar and segmental bronchi to reduce their muscle mass. This in turn reduces bronchial hyper-reactivity and bronchospasm – see Figure 4.
Bronchial thermoplasty is an option for patients with severe steroid-dependant asthma who are not candidates for treatment with Omalizumab (anti-IgE) or Mepolizumab (anti-IL5/eosinophils)
Pleurodesis and tunnelled pleural catheters
Malignant pleural effusion affects a significant number of patients with thoracic malignancies and has a major impact on quality of life. There are two options for management of malignant pleural effusion: pleurodesis or tunnelled pleural catheter insertion.
Pleurodesis is a procedure to induce symphysis between the parietal and visceral pleura, thereby abolishing the pleural space and preventing recurrence of effusion post drainage. It can be achieved by introduction of talc into the pleural space via chest tube or at thoracoscopy (medical or surgical). Additionally, if a surgical thoracoscopy (VATS) is performed, successful pleurodesis can be assisted by mechanical abrasion of the pleural surface.
The benefit of pleurodesis is that if successful, no further intervention is required to control the effusion for the life of the patient. However, it is only 70-80% successful and for those where the effusion reccurs, further procedures will be necessary. It also won’t work if the underlying lung does not re-expand fully post fluid drainage (trapped lung).
Tunnelled pleural catheter (or indwelling pleural catheter) placement involves inserting a soft small bore (**Fr) chest tube that is burrowed under the skin for ~5cm prior to skin exit (similar to a Hickman’s line). This means it can stay in place for many months with a reduced rate of infection (~5% annually). A nurse (or the patient’s family) can then drain pleural fluid on an as-need basis in the patient’s home). When not attached to a drain, the indwelling pleura catheter remains neatly folded against the skin with a cap to prevent fluid leakage.
The benefit of a tunnelled pleural catheter is that it gives patients control over their symptoms and will work in the setting of trapped lung (incomplete lung re-expansion post fluid drainage). The downside is that there needs to be ongoing management of the catheter for the life of the patient, to manage complications like tube blockage or infection. In ~40% of cases, there is a spontaneous pleurodesis over time allowing the indwelling pleural catheter to be removed.
Pleuroscopy (Medical thoracoscopy)
Pleuroscopy is a procedure performed under sedation with spontaneous ventilation (as opposed to surgical thoracoscopy which requires general anaesthesia and single lung ventilation). It is far more limited than surgical thoracoscopy, and is used to investigate undiagnosed exudative effusions. Usually a single access port is used in the axilla, and once pleural fluid is drained, a telescope is passed into the pleural space. This allows inspection of the parietal and visceral pleura with targeted biopsies of any lesions. If malignancy is seen, talc can be instilled at the end of the procedure to achieve pleurodesis or a tunnelled pleural catheter can be inserted.
We would like to thank Dr Salamonsen for taking the time to put together this very useful summary article and answering our questions. Users are welcome to leave comments below.
